New Study by Neuroscientists and Nephrologists Offers Clearest Views Yet On A Key Protein Found In Kidney and Brain, Opens Avenues to Treating Diseases
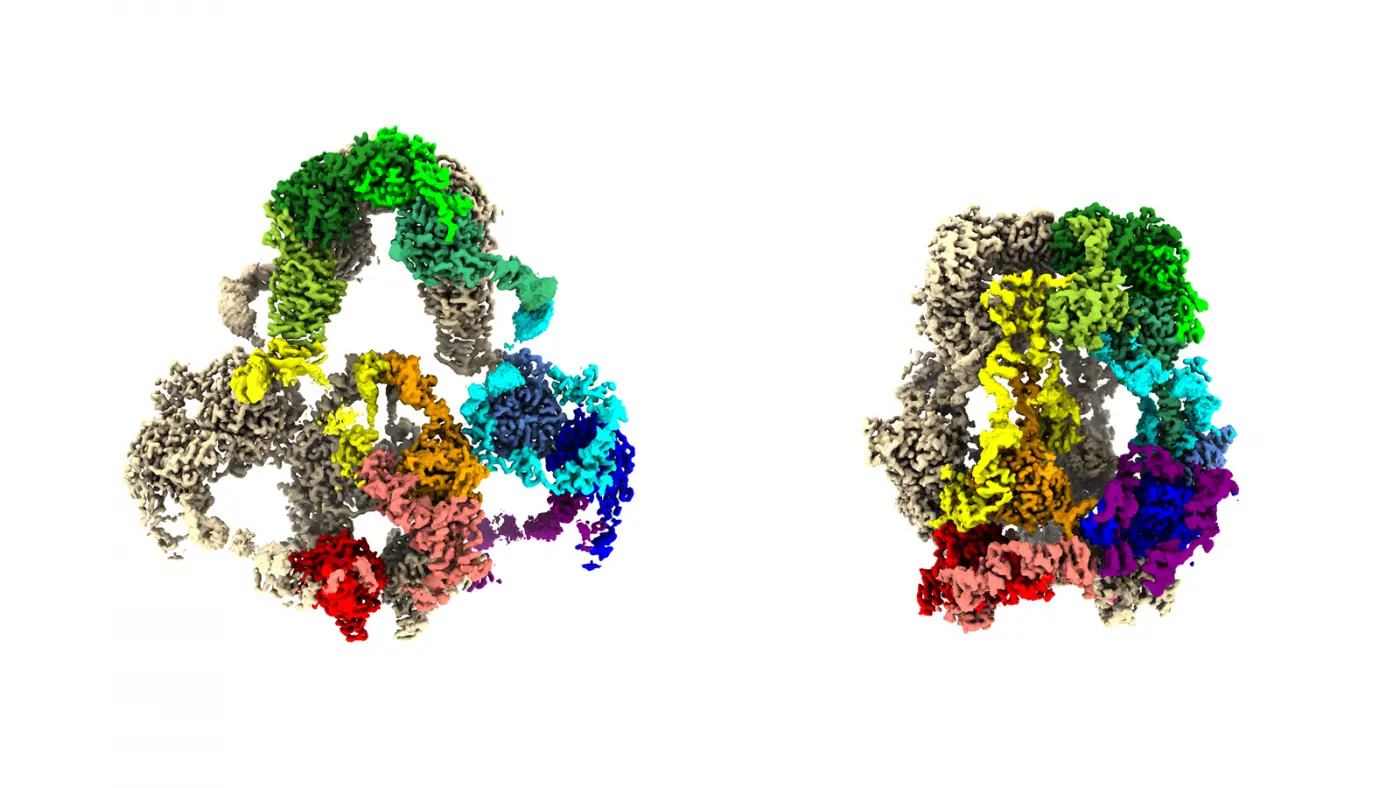
[ad_1]
The devil so normally is in the aspects. There are devils and angels in their aspects for proteins that orchestrate the molecular organization of existence, down to the proteins’ constituent atoms. It’s at that stage of structural trivialities where the harmony of health and disease, even life and dying, can pivot.

As unveiled by a cryo-electron microscope, the enormous protein megalin (LRP2) improvements condition. Graphic credit: A. Fitzpatrick, A. Beenken, L. Shapiro / Columbia’s Zuckerman Institute
Printed on the web in the journal Mobile, a collaboration of nephrologists and neuroscientists at Columbia University showed the value that emerges from unusual alliances. They and colleagues elsewhere expose for the first time a portrait of a life-and-loss of life protein with adequate clarity to eventually expose how it operates: as a minuscule ferry for molecular travellers that should cross practically a trillion mobile membranes in tissues and organs ranging from kidneys and brains to the internal ear and the lungs’ alveoli.
“With new mechanistic knowing of this vital protein, and how mutations in it can shut it down, we are hoping that follow-on study will uncover novel targets for managing kidney and mind diseases,” said Jonathan Barasch, MD, PhD, an specialist and clinician in urology and nephrology at Columbia’s Vagelos College or university of Physicians and Surgeons and a corresponding writer on the paper. “These new therapeutic openings are owing to the astounding protein constructions my Columbia colleagues Andrew Beenken, Anthony Fitzpatrick and Larry Shapiro have uncovered.”
Dr. Barasch and his coauthors imagine that the new substantial-resolution protein structures will level towards therapeutic prospects for treating illnesses as prevalent as acute kidney injury (influencing more than 4 million people for every calendar year in the United states), continual kidney condition (affecting some 800 million people all over the world) and Alzheimer’s disease (influencing an believed 32 million folks globally), and as scarce as Donnai-Barrow syndrome (affecting much less than 1,000 Us citizens), a genetic problem with several physical and cognitive penalties.
The protein is known as LRP2, a member of a loved ones of LRP proteins identified in creatures ranging from worms to people. In contrast to most proteins on mobile membranes, LRPs are massive, so a lot so that the researchers who found LRP2 in the early 1980s, Marilyn Farquhar and Dontscho Kerjaschki, dubbed it megalin. Some LRPs are created from extra than 4,600 amino acids, the molecular making blocks of all proteins.
In kidney cells, LRP2 is critical for recovering reusable molecules from filtered metabolic wastes from bodily fluids so the physique does not have to commit electrical power and means to make them all over again. For each and every of these cells, there are very likely tens of 1000’s of LRP2 proteins, dispersed on the area like seeds on a strawberry.
“The kidney is faced with recovering 99 per cent of the body’s salt and h2o that passes via the organ’s filters, and also with recovering 100 percent of little proteins that normally would dump into the urine and out of the entire body,” said Dr. Barasch. “There have been normal tips about how this recovery performs, but its specificity has now been solved.”
This is exactly where Dr. Barasch’s colleagues come in. One of them is Andrew Beenken, MD, PhD, a biochemist and nephrologist at Columbia and the paper’s to start with author. Membrane proteins like all those in the LRP loved ones are notoriously tricky to isolate, let by itself map out in depth. Dr. Beenken navigated a giant move all around that deadlock with an arduous method in which he deployed a bevy of biochemical approaches.
“When I initial listened to about what Jonathan and Andrew ended up arranging to do, I did not assume it would be doable,” mentioned co-corresponding author Lawrence Shapiro, PhD, a principal investigator at the Zuckerman Institute and a professor of biochemistry and molecular biophysics at Columbia’s Vagelos School of Physicians and Surgeons. Dr. Shapiro’s expertise in teasing out how a protein’s intricate composition begets its organic capabilities would confirm crucial in deciphering LRP2’s ferrying mechanism.
With a combine of benchtop skill, creative imagination and resolve, Dr. Beenken harvested adequate LRP2 protein from 500 mouse kidneys to solidify the protein into a sample of adequate measurement and purity for investigation with highly developed microscopy techniques. In his harvesting of the LRP2 molecules, Dr. Beenken pulled off a biochemical tour de power: capturing LRP2 proteins locked into two of their critical conformations, a essential laboratory feat for unveiling the protein’s equipment-like steps in cells.
This is where by a next huge investigation stage arrived in, this a single led by co-corresponding author Anthony Fitzpatrick, PhD, a leader in the area of cryogenic electron microscopy, which is primarily suited for learning substantial proteins and other biomolecules.
With a two-story, liquid-nitrogen-cooled, cryogenic electron microscope, Dr. Fitzpatrick and Dr. Beenken gathered vast amounts of structural details using Dr. Beenken’s challenging-gained LRP2 samples. Then, with the deft use of effective computational applications to make perception of the data, the researchers manufactured 3D protein buildings in close to-atomic element.
“We now have the ideal 3D maps of the LRP2 protein ever created,” reported Dr. Fitzpatrick, a principal investigator at the Zuckerman Institute and an assistant professor of biochemistry and molecular biophysics at Columbia’s Vagelos Higher education of Doctors and Surgeons. With all those maps, Dr. Shapiro could commence to tease out the outstanding system by which LRP2 operates in cells.
One particular of the mapped-out conformations captures the shape of the LRP2 when it resides on and within a mobile membrane. Which is exactly where the protein picks up molecular travellers from the liquid outside the house the cell–whether from the urine produced in the kidney or the liquid about mind cells. Amongst those people travellers are little proteins, which include tau and amyloid-beta (each implicated in Alzheimer’s ailment), insulin and ones that shuttle nutritional vitamins A and D close to cells.
The other LRP2 conformation is the a single the protein snaps into right after it gets enveloped in a little bit of mobile membrane and ferries off to areas within the cell. It is in these tiny capsules, endosomes, where the condition-shifting protein possibly permits its molecular passengers to be recycled intact for further use or to be deconstructed into reusable or disposable parts.
From this pair of LRP2 constructions, the group was ready to identify that the protein undergoes a machine-like toggle in between its passenger-embarking kind and its passenger-disembarking form. When LRP2 proteins are mutation-totally free, they do well in maintaining molecular balances in, for example, blood and mind tissue. But when there are even very small tweaks in LRPs’ great molecular anatomy, these proteins can contribute to condition.
In kidney cells, for 1, faulty LRP2 proteins renege on their ordinary process of retrieving proteins that would in any other case be shed in the urine. That can lead to a range of circumstances such as long-term kidney disorder, Donnai-Barrow syndrome and circumstances that are deadly in neonatal stages.
In the mind, LRP2 (and the connected LRP1) usually help very clear a wide range of toxins, amongst them tau protein fragments, which have very long been associated with Alzheimer’s ailment. But proteins of the LRP loved ones also have been proven to transfer these types of fragments among brain cells, most likely contributing to the disease course of action.
“You could consider that seeking to inhibit this from happening with a drug could be valuable,” reported Dr. Shapiro.
This is exactly where the electrical power of cryogenic-EM comes in sturdy.
“If you know particularly the atomic specifics of where by the tau binds, you may possibly really block that making use of both an antibody or a modest molecule,” mentioned Dr. Fitzpatrick. “Cryo-EM can get you to the level of detail you will need in get to get the job done on probable new therapies.”
“This is the starting of a extended street of discovery of how these LRP proteins get the job done and to new drug targets for a selection of disorders,” explained Dr. Beenken.
Supply: Columbia University
[ad_2]
Supply link







